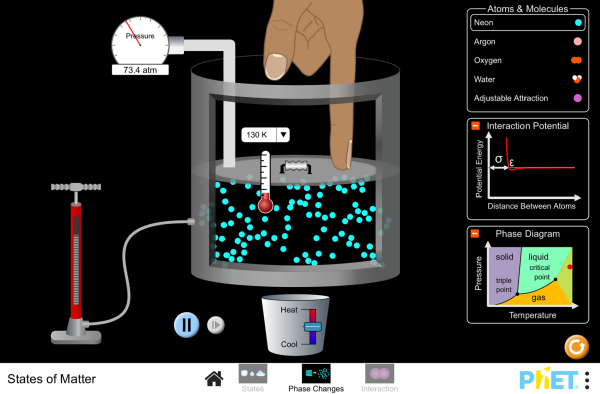
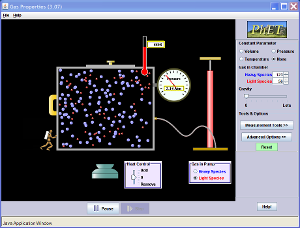
State Variables:
- volume (V), [m3]
- pressure (p), [Pa]
- mass (M), [kg]
- temperature (T) [K]: related to thermal energy (Eth)
- Derived: ρ = M/V (mass density) [kg m-3]
Molecules (分子) & Moles (摩爾mo2er3)
- N: Number of molecules [-]
- NA: 6.02 x 1023 [mol-1]
- n = N/NA [mol] (Why? For convenience)
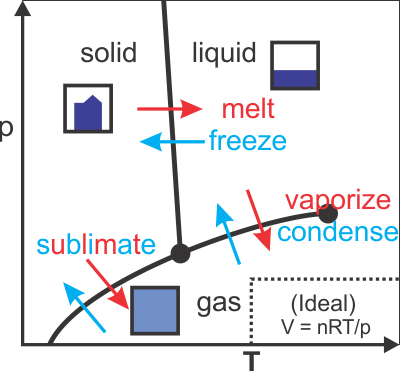
Basic phases of matter and phase diagrams
Ideal Gas Law & Processes
- Incompatable assumptions:
- molecules don't touch each other;
- gases in thermal equilibrium (requires contact)
- → Valid away from phase transition boundary (see phase diagram)
- Equation:.
- pV = n R T where R= 8.31 [J mol-1 K -1]
- pV = N kB T where kB=1.nt04 x 10-23 [J K -1]
- p-V diagrams (p,V → T, only concern Δ) for Quasi-Static Processes
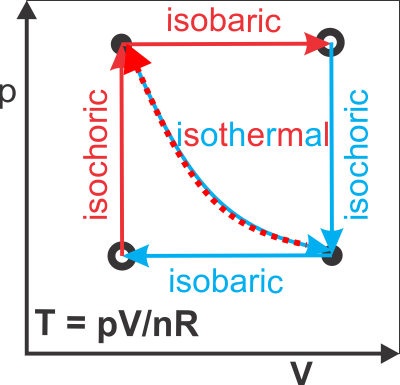
isochoric: Vf = Vi
isobaric: pf = pi
isothermal: Tf = Ti
Ref: A Macroscopic Description of Matter(Slides)
- Ref: Knight Version 3 Chapter 16
- Ref: Knight Version 4 Chapter 18
FAQ
- Why are the the quantities such are R and NA not simple numbers? ANS: This is due to history and the way units were first defined. For example, we could define a new unit of temperature the [bai]. If 10 [bai] = 8.31 [K] then R=10. (Water would freeze at 328.7 bai, and boil at 448 bai. I could then define a scale for daily use T [xiaobai] = T[bai] -328.7 so that the freezing point of water would be 0 [xiaobai] and boiling point would be 106.5 [xiaobai] ) Similarily we could define a new unit of mass, the [white] such that so that Avrogadro's number is 1024!
- Why can we only consider quasi-static processes in thermodynamics? ANS: P,V and T are only defined for thermodynamic equilibrium. If things move fast we leave this equilibrium → the state variables are not defined. i.e. T is not constant in the system, P is not constant everywhere. To draw a path on the pV diagram we need to go ∞ slow. See Quasistatic and Reversible Processes (Khan Academy) for more information.