Preamble (Review)
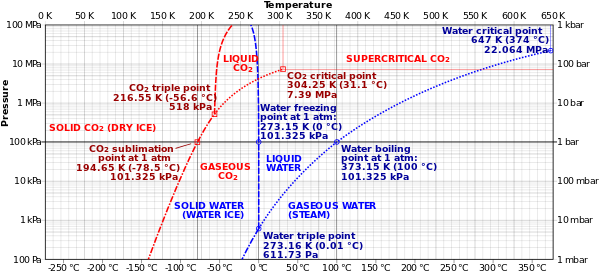
- Phase Diagrams (P vs T)
- Music - Quarks
- Review Lecture nt02
- Heat vs Temperature Julius Sumner Miller
Key Points
- Δ Esys = Δ K + Δ U + ΔEth = W + Q (1st law)
- K, U, E are nouns! W and Q are verbs!
- NB: W ≡ Wext (Work done on system, Heat transfered to system is positive)
- W = ∫ Fds = ∫ pAds = ∫ pdV
- Molar Specific Heat Capacity: Δ Eth= n C Δ T
C [J mol-1 K-1]
- Heat of Transformation: Q = ± ML [J kg-1]
(Phase change, Δ P = Δ V = 0)
- Ideal Gases
- pV Diagram (p vs V: 2 of 4 state variables, nRT=pV)
- Processes: Work (Path Dependent)
- isochoric: Vf = Vi → W = 0
- isobaric: pf = pi → W = -p Δ V
- isothermal: Tf = Ti → W = -nRT ln (Vf / Vi )
(NB: nRT=piVi =pfVf)
- Processes: Heat (Path Dependent)
- isochoric: W=0 → Δ Eth= Q = n Cv Δ T
- isobaric: → Q = n Cp Δ T → W = n (Cv - Cp) Δ T
- isothermal: Δ Eth=0
- adiabatic: Q=0 → Δ Eth= W = n Cv Δ T
- Heat-Transfer Mechanisms
- Conduction
- Convection
- Radiation
PowerPoint Presention
Knight Chapter 19 Work, Heat and 1st Law of Thermodynamics(Slides)
FAQ